8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Last updated 24 fevereiro 2025

Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed

Study Notes on Analytical Chemistry, EB 3247 - Analytical Chemistry - Nilai

8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N /10 NaOH

The Kjeldahl Titrimetric Finish: On the Ammonia Titration Trapping in Boric Acid

Estequiometria quimica, Ejercicios de Química

A sample of 0.50 g of an organic compound was treated according to Kjeldahl's method. The ammonia evolved was absorbed in 50 mL of 0.5 M H 2 SO 4. The residual

Methods of Purification of Organic Compounds: Crystallization, Distillation, and Chromatography, PDF, Chromatography

Reagents used during decomposition methods tested c/(mol L −1 ) Na 2 CO
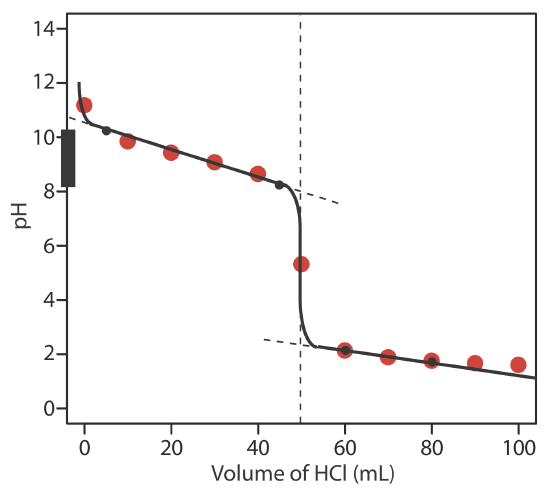
9.2: Acid–Base Titrations - Chemistry LibreTexts

0.2 gm of an organic compound was analysed by kjeldahl's method the ammonia evolved was absorbed






![Black Desert (2023) - Gameplay (PC UHD) [4K60FPS]](https://i.ytimg.com/vi/DxyatERtIiM/maxresdefault.jpg)







