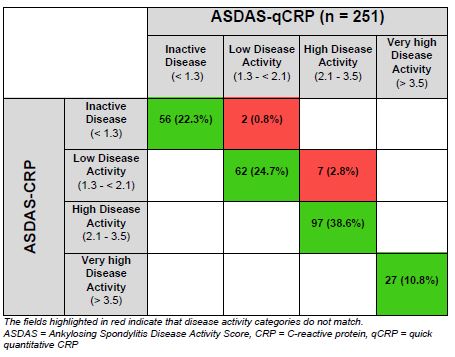
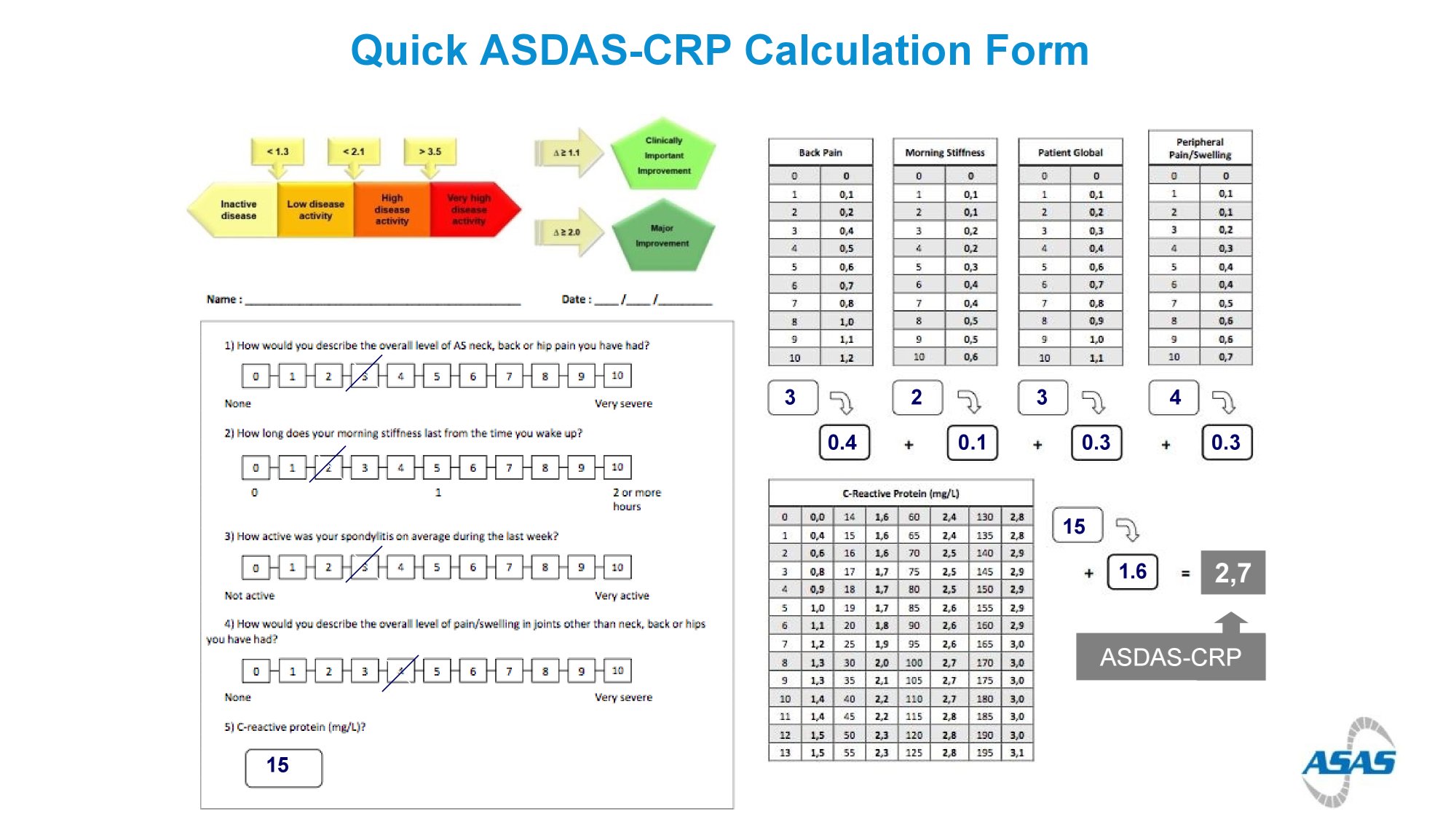
ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Last updated 26 abril 2025


PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis
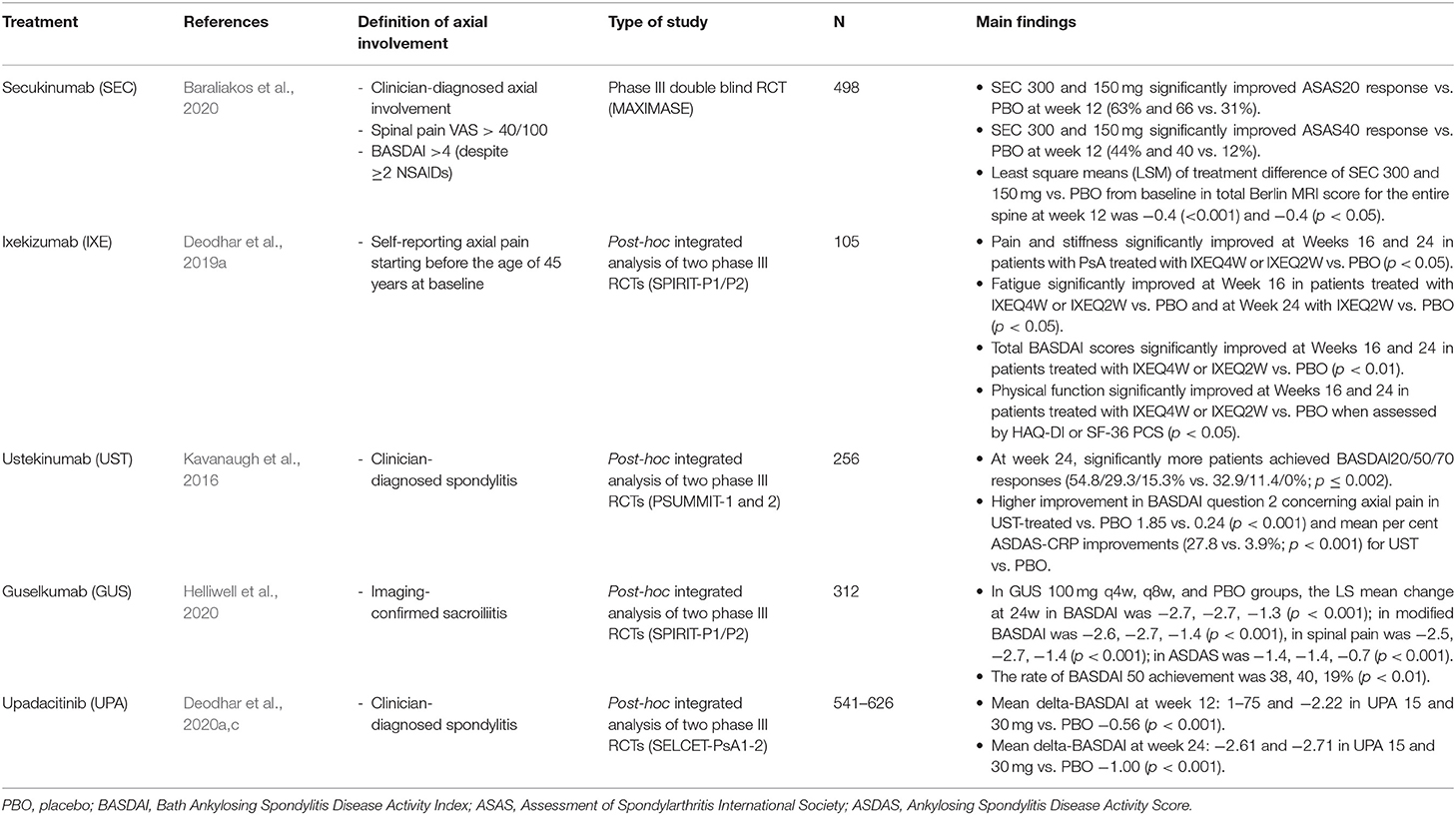
Frontiers Targeted Therapies in Axial Psoriatic Arthritis

Long-Term Safety and Efficacy of Ixekizumab in Patients With Axial Spondyloarthritis: 3-year Data From the COAST Program

Baseline characteristics of ASDAS ID and ASAS PR responders and

Disease Control Data, Ankylosing Spondylitis

Assessment of SpondyloArthritis international Society criteria for 20%

Long-term safety and clinical outcomes of certolizumab pegol treatment in patients with active non-radiographic axial spondyloarthritis: 3-year results from the phase 3 C-axSpAnd study

UCB Presents New Five-Year Data on BIMZELX® (bimekizumab-bkzx) in Ankylosing Spondylitis at ACR Convergence 2023

Disease Control Data, Ankylosing Spondylitis
Baseline predictors of (A) ASDAS ID and (B) ASAS PR at week 12 of
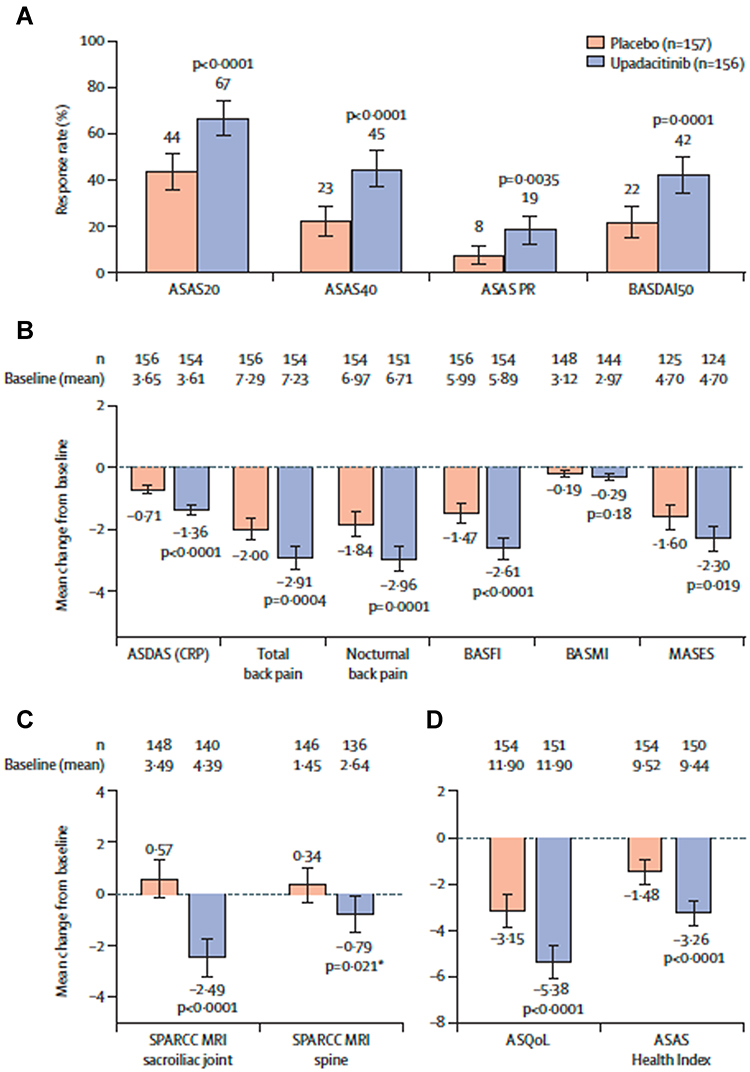
Management of axial spondyloarthritis
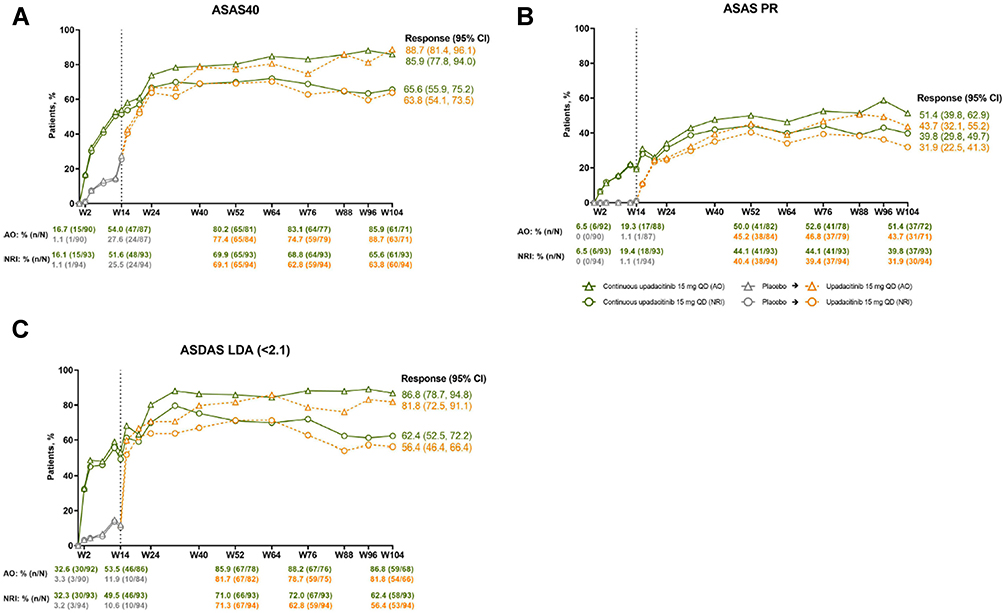
Management of axial spondyloarthritis
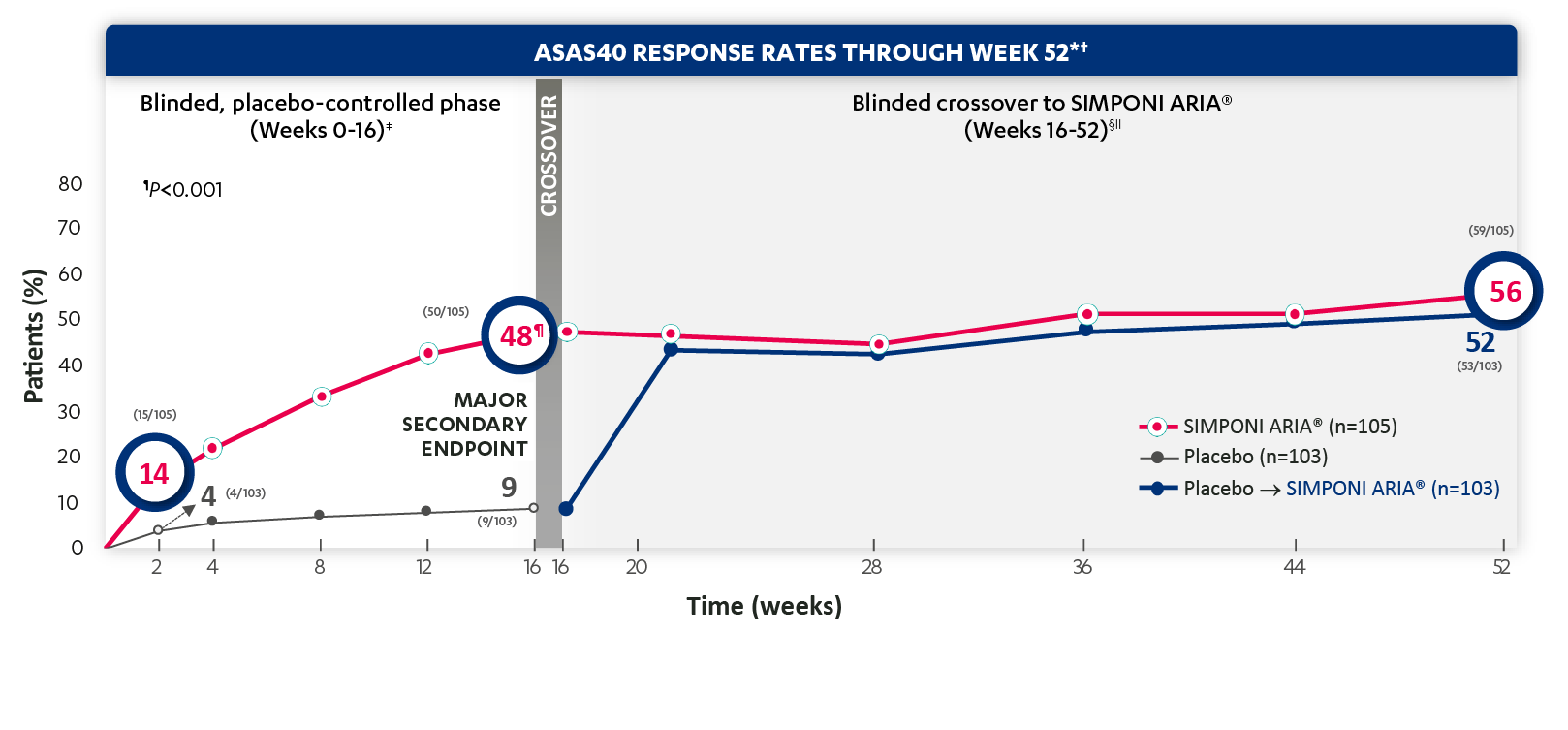
SIMPONI ARIA® Ankylosing Spondylitis: ASAS Response Rates

Baseline characteristics of patients included in the two treatment arms












![Kitsune] How to setup discord webhooks [AS2/AS3] - Tutorials - Solero](https://archive.solero.me/applications/core/interface/imageproxy/imageproxy.php%3Fimg=https:%252F%252Fimage.prntscr.com%252Fimage%252FT0y7_N68T-mY5WwLd-Oz-g.png&key=04b801ebfafaaf065e8201ea4c77d93afa36b746943efecca06f415f6cf9882a)

